Abstract
Aims & Objectives: Chimeric antigen receptor T (CAR-T) cell therapy has revolutionized the landscape of cancer therapy, obtaining significant efficacy especially in relapsed and refractory (R/R) B cell malignancies. In clinical patients, CAR-T cells are able to migrate into various organs and exert cytotoxicity on tumor cells, including the central nervous system (CNS), which serves as an immunological privileged site protected from peripheral blood. However, whether there is a possibility of the CNS relapse in patients with B-cell lymphoma who achieved complete remission (CR) after CAR-T cell therapy has not been reported yet. We aim to describe the features of the CNS relapse after CAR-T cell therapy in B-cell lymphoma, and try to identify correlated factors and propose potential management strategy in the upcoming research.
Patients/Materials & Methods: We preliminarily reviewed 12 patients (5 presented) who received CAR-T cell therapy for the treatment B-cell lymphoma in our center and Shenzhen University General Hospital. We compared the baseline of patient characteristics before CAR-T cell therapy, described the clinical response and efficacy during the treatment, and reported the occurrence of CNS relapse after achieving CR.
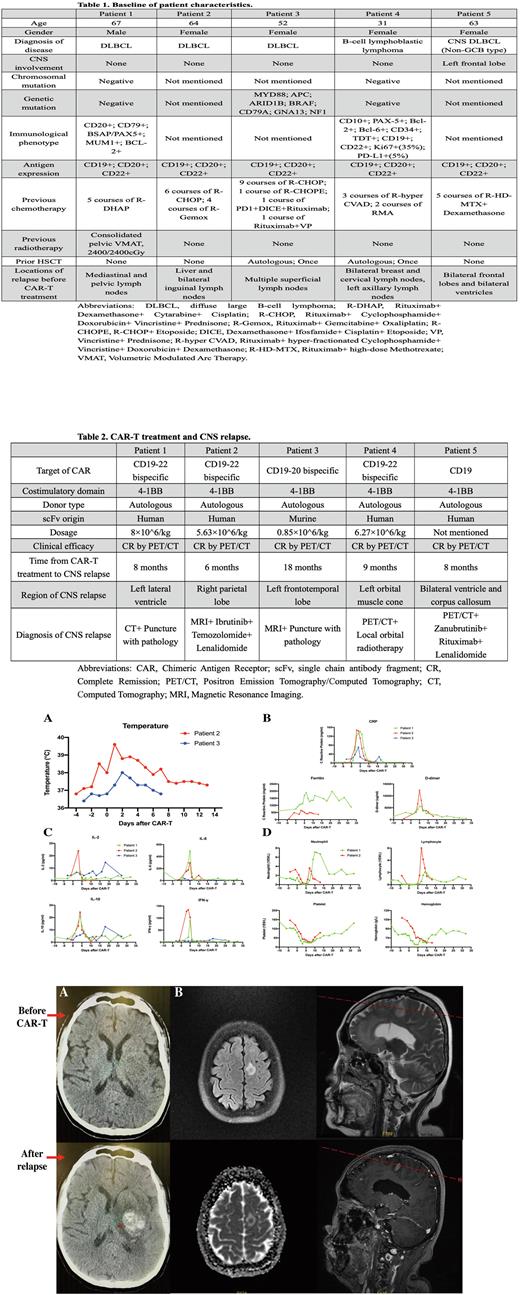
Results: All 5 patients were heavily pre-treated with multiple lines of chemotherapy prior to CAR-T cell therapy, with 2 of them received additional autologous hematopoietic stem cell transplantation (HSCT) (Table 1). All patients suffered from previous relapse in multiple peripheral lymph nodes, with 2 of them involved in other organs like liver and breast, and 1 of them involved in the CNS. Three patients received CD19-22 bispecific CAR-T product, 1 patient received CD19-20 bispecific CAR-T product, and 1 patient received CD19 CAR-T product (Table 2). All patients responded well with tolerable toxicities by monitoring body temperature, inflammation markers, cytokines, and hemogram, and all achieved CR by PET/CT after CAR-T treatment (Figure 1). All patients eventually developed isolated CNS relapse with different locations, respectfully, which was confirmed by imaging along with pathological diagnosis or targeted drug treatment (Figure 2).
Discussion & Conclusion: We first reported the CNS relapse after CAR-T cell therapy in patients with B-cell lymphoma, suggesting that routine monitory is required for patients who achieved remission after CAR-T treatment to manage the CNS involvement in time. If possible, additional application of PD-1 monoclonal antibody, BTK inhibitors or other immunomodulatory drugs that can function in the CNS might prevent the CNS relapse without affecting CAR-T cell function.
Conflict of Interest Statement: No conflict of interest to disclose.
Figure 1. Clinical responses and hematological toxicities during CAR-T treatment among 3 patients with CNS relapse after therapy. (A) Body temperature. (B) Levels of inflammation markers including C reactive protein (CRP), ferritin, and D-dimer. (C) Levels of cytokines, including interleukin (IL)-2, IL-6, IL-10, and interferon-γ (INF-γ). (D) Hemogram (ie. Neutrophils, lymphocytes, platelet, and hemoglobin).
Figure 2. Central nervous system relapse among 3 patients after CAR-T cell therapy. (A) Patient 1 with one single focus in left lateral ventricle confirmed by CT plus puncture with pathology (not shown). (B) Patient 3 with one focus in left frontotemporal lobe confirmed by MRI plus puncture with pathology (not shown).
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.